Support for Phase 1 Clinical Trials
UVA Cancer Center’s achievement of “Comprehensive” status—a National Cancer Institute designation reserved for only the country’s top cancer care and research institutions—is a testament to its robust and growing research enterprise. Nearly every week in labs across the University, scientists in various disciplines are making discoveries that improve our understanding of blood cancers, melanoma, glioblastoma, breast cancer, gastrointestinal cancers, and many other forms of this insidious disease. They are cellular and molecular biologists, immunologists, biomedical engineers, physiologists, chemists, geneticists, and more, and they’re increasing our foundational knowledge of cancer diagnosis, prevention, and treatment.
Meanwhile, the Cancer Center is running hundreds of clinical trials to test newly developed treatments on patients. These are experimental drugs and therapies first discovered and developed at UVA or by industry partners with the potential to extend and improve the quality of current and future cancer patients’ lives in Virginia and beyond. Until recently, however, the Center had limited capacity to manage a primary stage in the development and availability of new cancer treatments: phase 1 clinical trials.
Cancer clinical trials typically have four phases. Phase 1 trials are the earliest to establish a drug’s safety and dosing and are often the first-in-human tests of new therapies after experiments on cells or animals. Because of their high risks, phase 1 clinical trials are labor- and resource-intensive. They require enrolling only small groups of patients at a time and awaiting sufficient safety intervals in between. They require investigations of multiple tumor types and much more flexible and dynamic scheduling and intensive monitoring of these patients during treatment sessions that can last 8-12 hours.
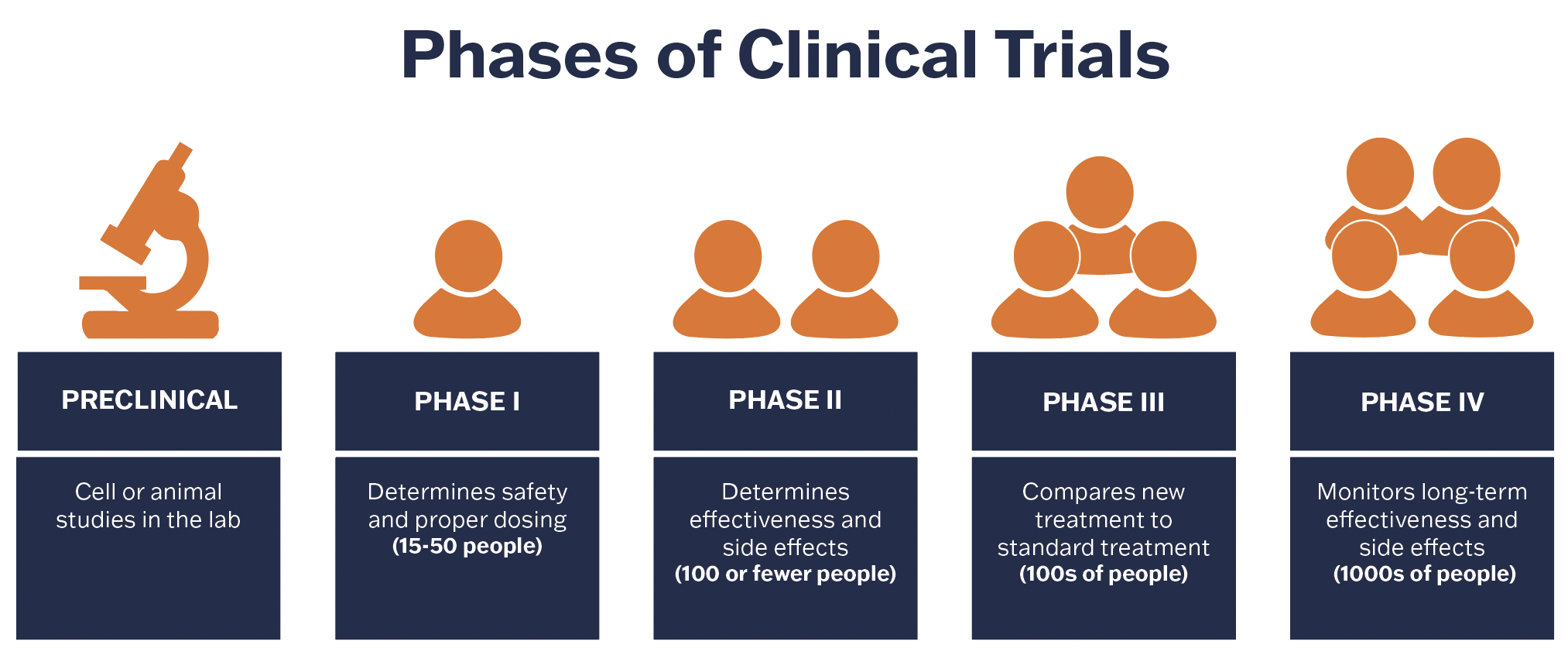
Currently, there are relatively few phase 1 clinical trials offered at UVA Cancer Center, but thanks to new Cancer Center investments and private support, that is poised to change. In June 2022, Dr. Tom Loughran, director of UVA Cancer Center, identified establishing a fully realized Phase 1 Clinical Trials Program as a natural progression and crucial next step in the UVA Cancer Center's translational research goals and announced the appointment of Dr. Matthew Reilley as the program’s inaugural director. Dr. Reilley is a GI medical oncologist and phase 1 clinical investigator who helped grow the Center's phase 1 clinical trials portfolio and significantly increased the Center's national presence as a phase I center.
In his announcement, Dr. Loughran said, "With the recent achievement of Comprehensive status, we believe this is an opportune time to formalize this program to further encourage and enable the translation of UVA science into the clinic and continue to develop our rapidly growing relationship with a variety of pharma and biotech partners."
If You Build It
For many patients with advanced cancer, the experimental treatments tested in phase 1 clinical trials offer new, and sometimes last, hope. Due to the complexity, risk, and expense of these trials, however, participation slots are few and fill up quickly. Dr. Reilley said he receives numerous calls each week from patients seeking access to experimental drugs and treatments.

“We want to get new therapies into the clinic and into patients as quickly and efficiently as possible,” said Dr. Reilley, “especially for patients who have exhausted other options.”
Doing so at UVA will take more time, funding, and outreach. A successful Phase 1 Clinical Trials Program requires dedicated programmatic leadership (physicians, nurses, and research coordinators), and those leaders must have adequate time and funding for program development and management. For example, in addition to seeing his own patients and overseeing the transition of the Center’s current phase 1 trials to this new umbrella program, Reilley is busy building relationships with oncologists across Virginia to identify patients who could benefit from UVA’s trials. Cultivating these community partnerships will be key to establishing a fully realized Phase 1 Clinical Trials Program at UVA and helping even non-UVA providers care for their patients by helping to match those patients with the experimental trials and treatments that could extend or save their lives.
Many peer institutions with strong phase 1 clinical trials programs also have committed infusion spaces for treating phase 1 patients—from dedicated infusion chairs to dedicated infusion units with support staff. Investing in dedicated staffing, infrastructure, and foundational programming is where private philanthropy can significantly impact the development of a successful Phase 1 Clinical Trials Program at UVA.
Critical Support
Philanthropy is vital to establishing a Phase 1 Clinical Trial Program at UVA Cancer Center and ensuring its long-term success—something two recent donors immediately recognized. Both took advantage of the Cancer Center’s gift-matching program to double the impact of their support.
“When Dr. Loughran identified establishing a Phase 1 Clinical Trials Program as a top priority for the Cancer Center, I knew I wanted to contribute,” says Adele Hoffmeyer, who is a long-time supporter of UVA Cancer Center with her husband, Rich, and chair of the center’s Advisory Board.
The importance and urgency of building phase 1 trials capacity similarly resonated with donor and Advisory Board member Colleen J. Grant. “With a Phase 1 Clinical Trials Program, the Cancer Center can offer hope to many more patients and speed UVA’s development of new cancer treatments. It’s incredibly exciting and inspiring,” she says.
Another recent gift that is critically important to the Cancer Center’s emerging Phase 1 Clinical Trials Program is Paul and Diane Manning’s $100 million contribution toward UVA’s building of an Institute of Biotechnology. This institute will advance UVA’s development of new medicines and therapies, particularly cellular and gene therapies and treatments for cancers that are prominent in the Cancer Center’s catchment area, such as colon, breast, and lung. The Cancer Center’s Phase 1 Clinical Trials Program will offer the first step in validating these new cancer-related treatments and getting them into patients.
“We have an excellent track record with industry partners, but what we really want to grow is internally developed therapies,” said Reilley. “This new biotech institute will enable the initial translational steps in manufacturing new treatments at UVA that we can test in Phase 1 trials. That’s the real excitement.”
Looking to the Future
As for growth targets, Reilley says the hope and intention are to double the size of the Center’s Phase 1 Clinical Trials Program over the next three to five years.
“We’d like to enroll 100 cancer patients annually in phase 1/early-phase trials,” he said, “with at least half of those rooted in UVA-based science.”
In many ways, Reilley says building a Phase 1 Clinical Trials Program epitomizes the notion of investing in hope.
“If we can push the limits to find better therapies and give patients the opportunity to receive them, even if it's not going to work for everyone, that’s a success,” he says.
This article was authored by Katherine Ludwig.
Learn more about UVA Cancer Center’s Phase 1 Clinical Trials and how you can support this vital program by contacting Corley Raileanu, Executive Director of Development, Cancer Programs, at corley@virginia.edu or call us at (434) 924-8432 or (800) 297-0102.

